

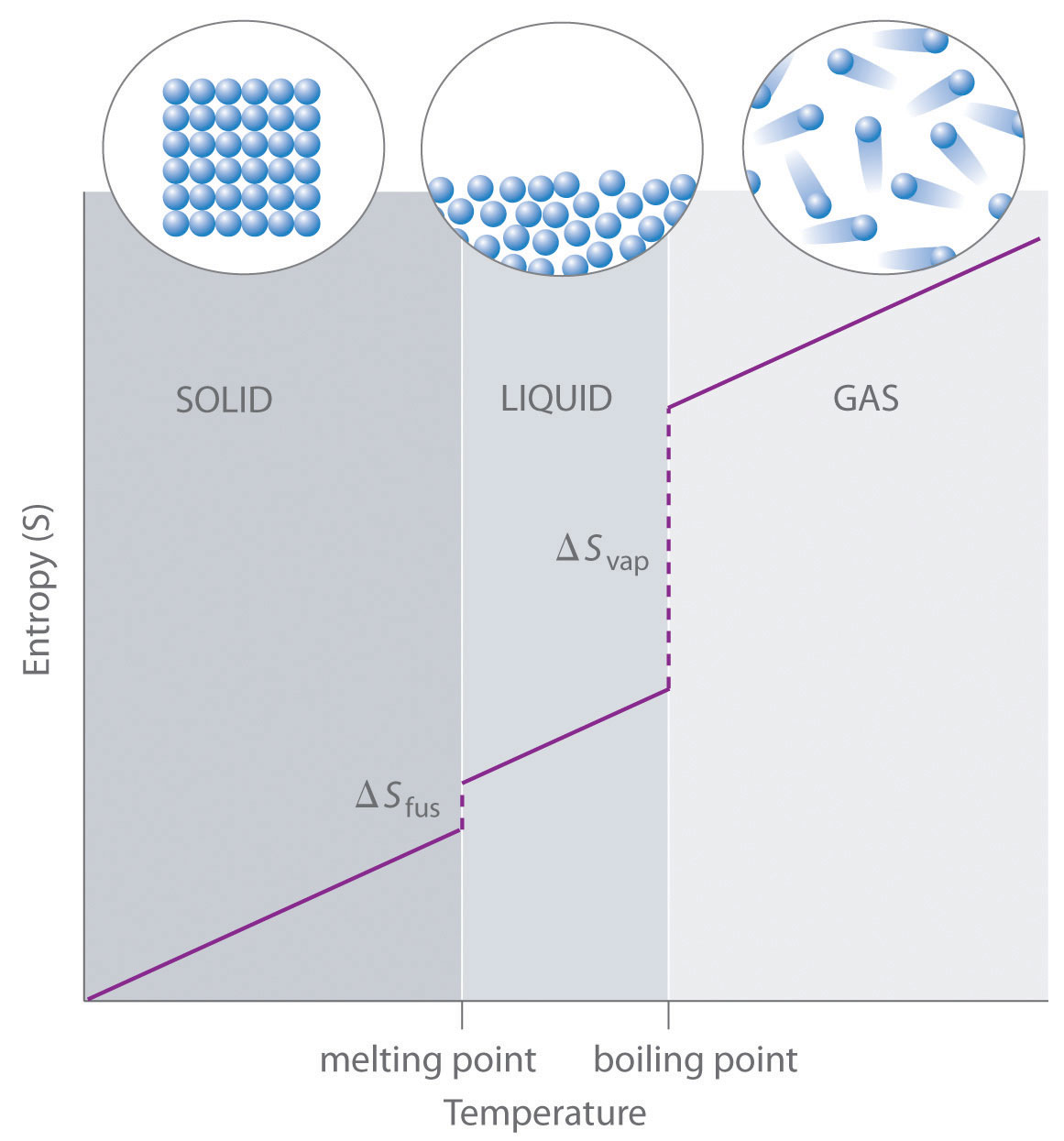
Any heat energy remains controlled as well, with certain cold pockets, like the refrigerator, and hot pockets, like the oven, with different temperatures that don’t spread to the rest of the house. Any mechanical energy of motion, such as water and gas moving through pipes, remains tightly controlled and directed. The mass that makes up the house is ordered and exact to form the walls and furniture. Low EntropyĪ system low in entropy involves ordered particles and directed motion. The letter “S” serves as the symbol for entropy.Īs we’ll find out in a later section, entropy has a lot of use for chemists and physicists in determining the spontaneity of a process. This means that as a system changes in entropy, the change only depends on the entropies of the initial and final states, rather than the sequence (“path”) taken between the states. Importantly, entropy is a state function, like temperature or pressure, as opposed to a path function, like heat or work. Topics Covered in Other ArticlesĮntropy is a measure of how dispersed and random the energy and mass of a system are distributed. Therefore, any change that results in a higher temperature, more molecules, or a larger volume yields an increase in entropy.In this article, we discover the meaning of entropy and its importance in thermodynamics, in both the universe and within a system. As well, increasing the volume of a substance increases the number of positions where each molecule could be, which increases the number of microstates. Increasing the number of molecules in a system also increases the number of microstates, as now there are more possible arrangements of the molecules. This increases the number of microstates possible for the system. We can estimate changes in entropy qualitatively for some simple processes using the definition of entropy discussed earlier and incorporating Boltzmann’s concept of microstates.Īs a substance is heated, it gains kinetic energy, resulting in increased molecular motion and a broader distribution of molecular speeds. A process that gives an increase in the number of microstates therefore increases the entropy. Microstates is a term used to describe the number of different possible arrangements of molecular position and kinetic energy at a particular thermodynamic state. Where k is the Boltzmann constant (1.38 × 10 −23 J/K), and W is the number of microstates. He developed an equation, known as the Boltzmann equation, which relates entropy to the number of microstates ( W): Ludwig Boltzmann (1844–1906) pioneered the concept that entropy could be calculated by examining the positions and energies of molecules. The Boltzmann Equation Figure 18.2 “Ludwig Boltzmann” Figure 18.1 “Two-Atom, Double-Flask Diagram.” When the stopcock is opened between the flasks, the two atoms can distribute in four possible ways. Thus we can say that it is entropically favoured for the gas to spontaneously expand and distribute between the two flasks, because the resulting increase in the number of possible arrangements is an increase in the randomness/disorder of the system. If we increased the number of atoms, we would see that the probability of finding all of the atoms in the original flask would decrease dramatically following (½) n, where n is the number of atoms. The likelihood of all atoms being found in their original flask, in this case, is only 1 in 4.

If we were to take snapshots over time, we would see that these atoms can have four possible arrangements.
Entropy chemistry free#
When the stopcock is opened, both atoms are free to move around randomly in both flasks. At first, both atoms are contained in only the left flask.

In this system, we have placed two atoms of gas, one green and one blue. The Molecular Interpretation of EntropyĬonsider the following system, where two flasks are sealed together and connected by a stopcock (see Figure 18.1 “Two-Atom, Double-Flask Diagram”). These definitions can seem a bit vague or unclear when you are first learning thermodynamics, but we will try to clear this up in the following subsections. But what exactly is entropy? Entropy is typically defined as either the level of randomness (or disorder) of a system or a measure of the energy dispersal of the molecules in the system. The second law of thermodynamics states that a spontaneous process will increase the entropy of the universe. To assess the spontaneity of a process we must use a thermodynamic quantity known as entropy ( S). To be able to estimate change in entropy qualitatively.To gain an understanding of the Boltzmann equation and the term microstates.To gain an understanding of the term entropy.


 0 kommentar(er)
0 kommentar(er)
